Medical Chemistry
Graduate School of Medical Science
Kyoto Prefectural University of Medicine

Research
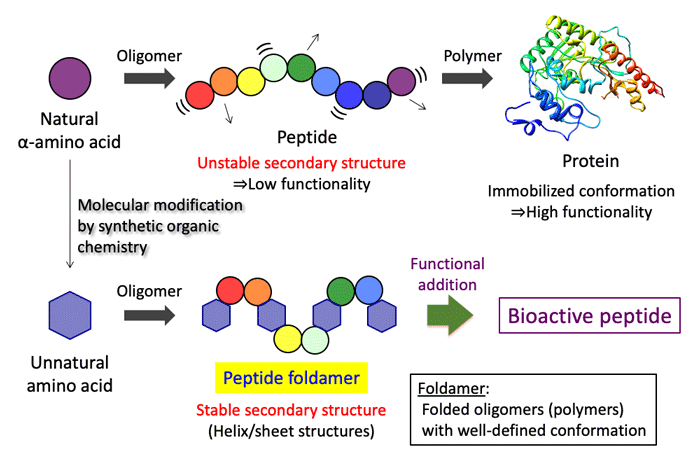
I. Study on peptide foldamers
Middle-sized drugs are attracting attention as the third drug after low-molecular-weight drugs and biopharmaceuticals. Peptides are promising materials for middle-sized drugs, because they can be chemically synthesized and chemical libraries can be easily constructed. However, even if peptides show their functions in vitro, it dose not mean that they can fully exert their functions in vivo. Reasons for this include the inability to form a stable secondary structure (conformation) in the body and the fact that it is easily decomposed by abundant hydrolases. As an approach to solve these problems, we are engaged in research of peptide foldamers utilizing unnatural amino acids. Foldamers are folded oligomers or polymers with a well-defined conformation. A stable secondary structure enables peptides to show sufficient function in the body and utilization of unnatural amino acids leads to difficulty to be recognized by hydrolases, resulting in persistence of function. We are aiming for application to functional peptides by independently designing and synthesizing unnatural amino acids and establishing a methodology that can freely control the peptide secondary structure.
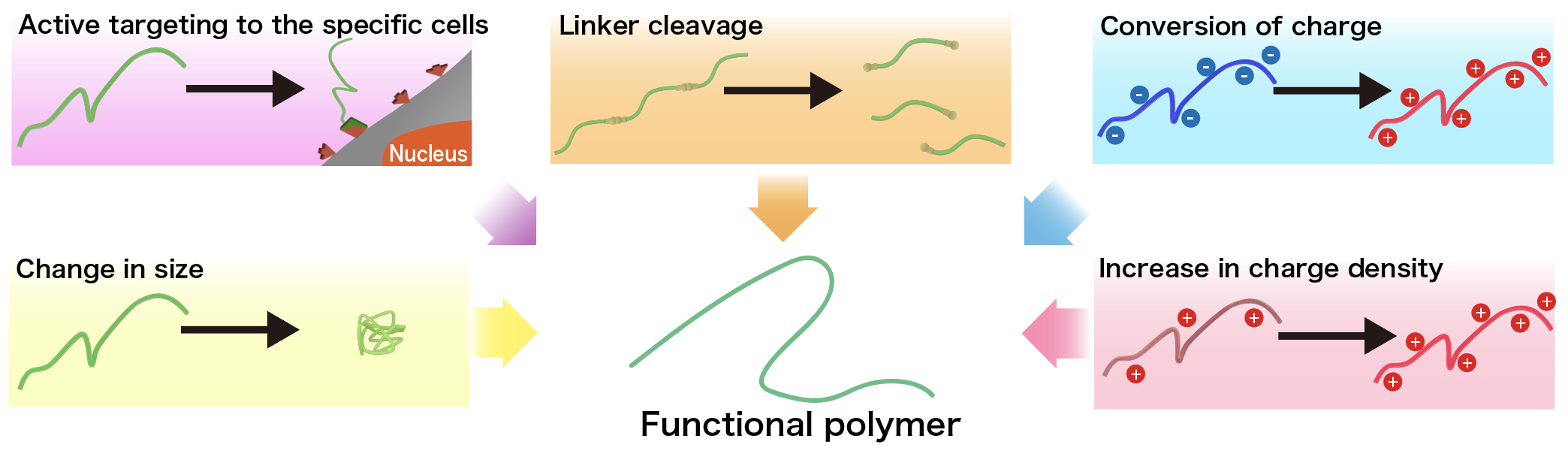
II. Development of functional polymers that recognize biological microenvironments
Each microenvironment in our body has a different pH, redox potential, enzyme activity, and metabolite concentration. By designing molecules that can recognize these environmental differences (stimuli-responsive elements), it is possible to express site-specific functions (e.g., medicinal effects). Examples of such stimuli-responsive elements include linker cleavage, conversion and density change of electroc charges, size changes, and adsorption to specific tissues (or cells). In this regard, the ability to introduce multiple stimuli-responsive elements into a single molecule would enable sophisticated molecular programs that can recognize different microenvironments in a stepwise manner to express their functions. Since polymers have many reaction sites in a single molecule, multiple stimulus-responsive elements can be introduced into a single molecule without any restriction on combinations, and are considered to be an indispensable material for next-generation pharmaceuticals. We are challenging to construct innovative polymers for drug discovery, starting from the design and chemical synthesis of new polymers, followed by physicochemical evaluation and various biological evaluations.
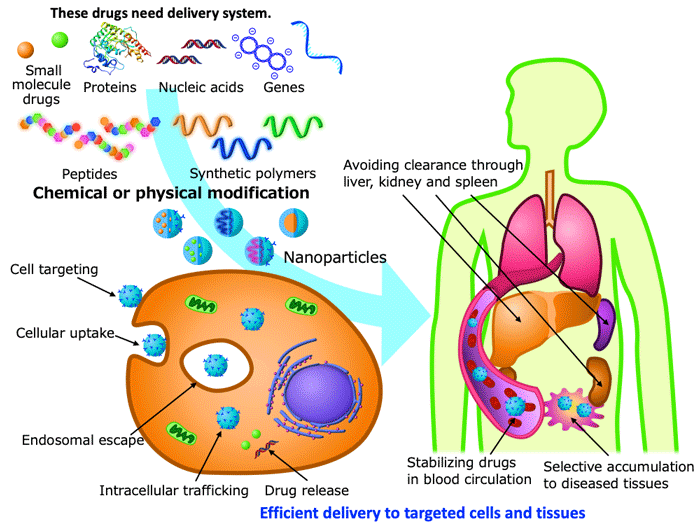
III. Drug delivery system (DDS)
DDS aims at drug functioning in a proper period, at a proper place and at a proper amount, and has developed as a methodology for quantitative, temporal and special control of drug biodistribution. Recently, DDS attracts growing attention as a methodology for delivering proteins, nucleic acids and genes to targeted cells and tissues in a safe and efficient manner. In our DDS researches, we use peptide foldermers and synthetic polymers mentioned above for precisely controlling drug distribution at the levels of cells, tissues and whole bodies. We devote our vigorous effort to curing intractable diseases by developing DDS for gene therapy and nucleic acid therapeutics, and also to developing research tools for cell and chemical biology.
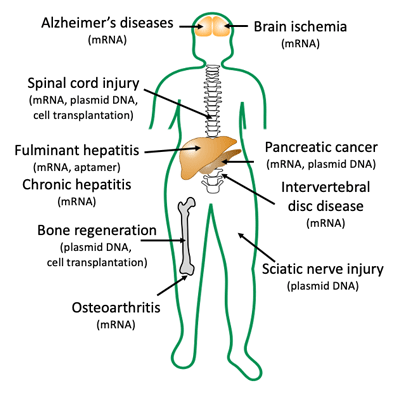
IV. Therapeutic applications
Therapeutic experiments using small animals are indispensable for clinical development of novel technologies. The therapeutic experiments often provide valuable findings that cannot be obtained by reporter assays. For example, toxicological properties of newly developed materials can negatively influence the therapeutic processes even at the level undetectable in common toxicological analyses. After the therapeutic studies, we proceed into clinical development in some cases or move back to basic study with feedback from the therapeutic studies. We succeeded in treating animal models of various diseases throughout the body, by using plasmid DNA, mRNA, siRNA, DNA aptamer, and cell transplantation. These experiences will allow us for smooth transition from basic development of new technologies in this laboratory to therapeutic experiments and further to clinical development.